Stage 1: Strategic Blueprint
Stage 1: Strategic Blueprint is focused on helping you build a tailored roadmap for your clinical trial success
In the Strategic Blueprint stage, we develop a tailored roadmap for your clinical trial success. Our process includes a vendor landscape assessment for clinical trials, sourcing qualified partners, estimating costs and timelines while accounting for risks, and ensuring compliance throughout. This blueprint serves as a comprehensive guide to streamline every phase of your trial.
By creating a strategic blueprint, we ensure that you are prepared to move forward with confidence. Get a head start with strategic planning for your clinical trials.

Vendor Strategy
Effective Strategies That Drive Operational Readiness
We help you optimize your vendor strategy with expert insights and consulting. We ensure you’re operationally ready, establishing clear timelines and budgets. Our independent, unbiased approach provides you with the knowledge to make informed decisions, address investor concerns, and reduce risks in your clinical trials.
Market Scan
Make Informed Decisions with Our Comprehensive Market Scan Service.
Our Market Scan service provides a tailored, in-depth analysis to identify the best-fit vendors for your clinical trial. We combine expert insights with thorough evaluation methods to give you a ranked shortlist of vendors that align with your specific needs. This approach ensures you select partners who will deliver maximum value, reduce risks, and drive clinical success.

The Seuss+ Advantage
With over 14 years of biotech and pharma experience, VRMM offers a proven, five‑step framework that enhances vendor performance, mitigates trial delays, and protects quality. We’re not passive advisors, we roll up our sleeves and execute alongside your team, ensuring hands‑on control all the way through.
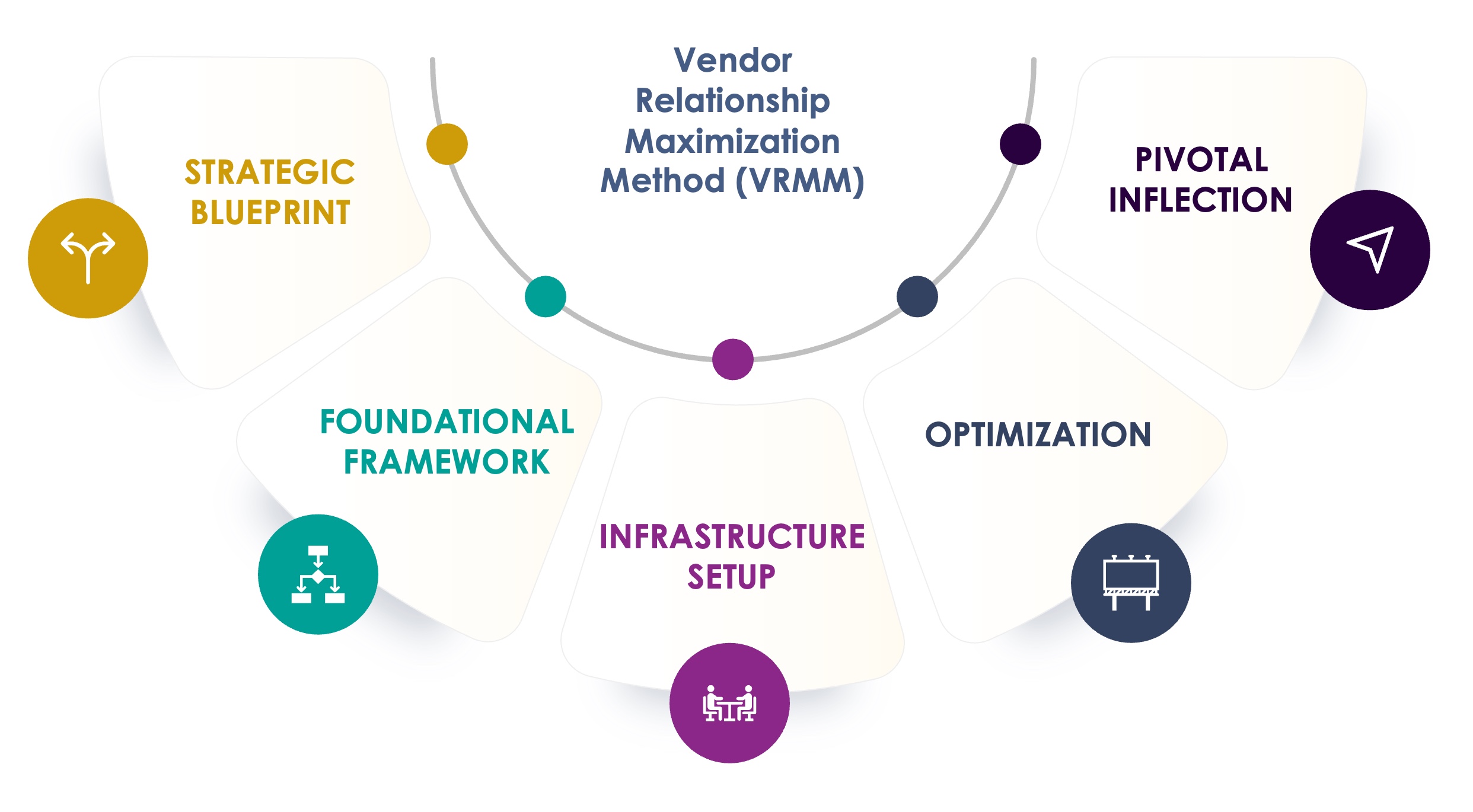
Develop a vendor strategy that aligns with your goals
- Vendor Strategy
- Market Scan
- Quality Management System (QMS)
Establish robust systems to ensure seamless vendor collaboration
- Vendor Selection
- Contract Negotiation
- Vendor Qualification (GCP & GMP)
- Systems Validation Consulting
Build an operational foundation for effective vendor management
- Study Risk Management Setup
- Governance & Reporting
- Vendor Oversight
- Clinical System Analysis
- Sponsor & Vendor Team Dynamics Workshop
- Clinical System Implementation and Oversight
Refine vendor performance to maximize efficiency and your strategic outcomes
- Vendor Management
- Budget & Change Order Management
- Risk Management
- Conflict & Collaboration Solutions
Execute key adjustments to elevate vendor partnerships and outcomes
- Inspection Readiness

