Stage 2: Foundational Framework
Stage 2: Foundational Framework is Focused on Alignment Between Sponsors and Vendors
In the Foundational Framework stage, we provide expert guidance to ensure alignment between sponsors and vendors, supporting your asset development and clinical trials with partners that match your unique needs and company culture. We take a hands-on approach to contract negotiations, safeguarding your interests by aligning vendor terms with your project goals. Our team identifies opportunities to streamline operations, close potential gaps, and establish robust systems for seamless vendor collaboration, ensuring your trials are set up for success from the outset.

Vendor Selection
Elevate Your CRO Selection Process
Keep your trials on track and on time, and your budget under control with a comprehensive Vendor Selection Process. Whether you’re looking for a lab or logistics supplier, a specialty provider, or a full-service CRO, we can support you from alignment to award. Our vendor selection experts follow our proven process, working efficiently to help you meet the study startup timeline and enabling you to focus on what matters most: running your trial.
Contract Negotiation
Create Clarity, Save Resources, and Build a Solid Foundation for your Vendor Relationship.
With our Contract Negotiation Service, you benefit from over a decade of life science and business expertise, so you can apply the inside knowledge we have gained working with the major CROs and CDMOs. Your needs dictate our approach: we build a custom process using proven elements, including a contract template that puts you in control of the negotiation.


Vendor Qualification (GCP & GMP)
Vendor Qualification Tailored to the Needs of Your Trial
We conduct fit-for-purpose vendor qualification through expert audits, qualification questionnaires, SOPs, and comprehensive document reviews to confirm that your vendors are fully qualified for the services they provide.
Our Vendor Qualification service gives you confidence that all vendors used for your clinical trials are qualified for their services. We offer tailored evaluations using audits, visits, questionnaires, and reviews of SOPs and relevant documentation.
Our experienced experts provide a thorough assessment, identifying potential risks and aiding in informed vendor management decisions.
Systems Validation Consulting
Expert Guidance for Navigating System Validation
Experts guide you through the intricate process of system validation. We help you set up required audits, craft effective Standard Operating Procedures (SOPs), and align on a pragmatic strategy for how your organization will approach validation. This enables you to demonstrate how you have ensured your computerized system is fit for purpose, which is a regulatory requirement. Our consulting expertise helps to ensure your validation process is well-planned, documented, and aligned with industry best practices, increasing the likelihood of successful outcomes. .

The Seuss+ Advantage
The Seuss+ Vendor Relationship Maximization Method (VRMM) is a proven process, honed over more than a decade, that ensures your vendors perform at their best. A systematic approach that helps reduce trial delays, mitigate risks, and drive your clinical development success.
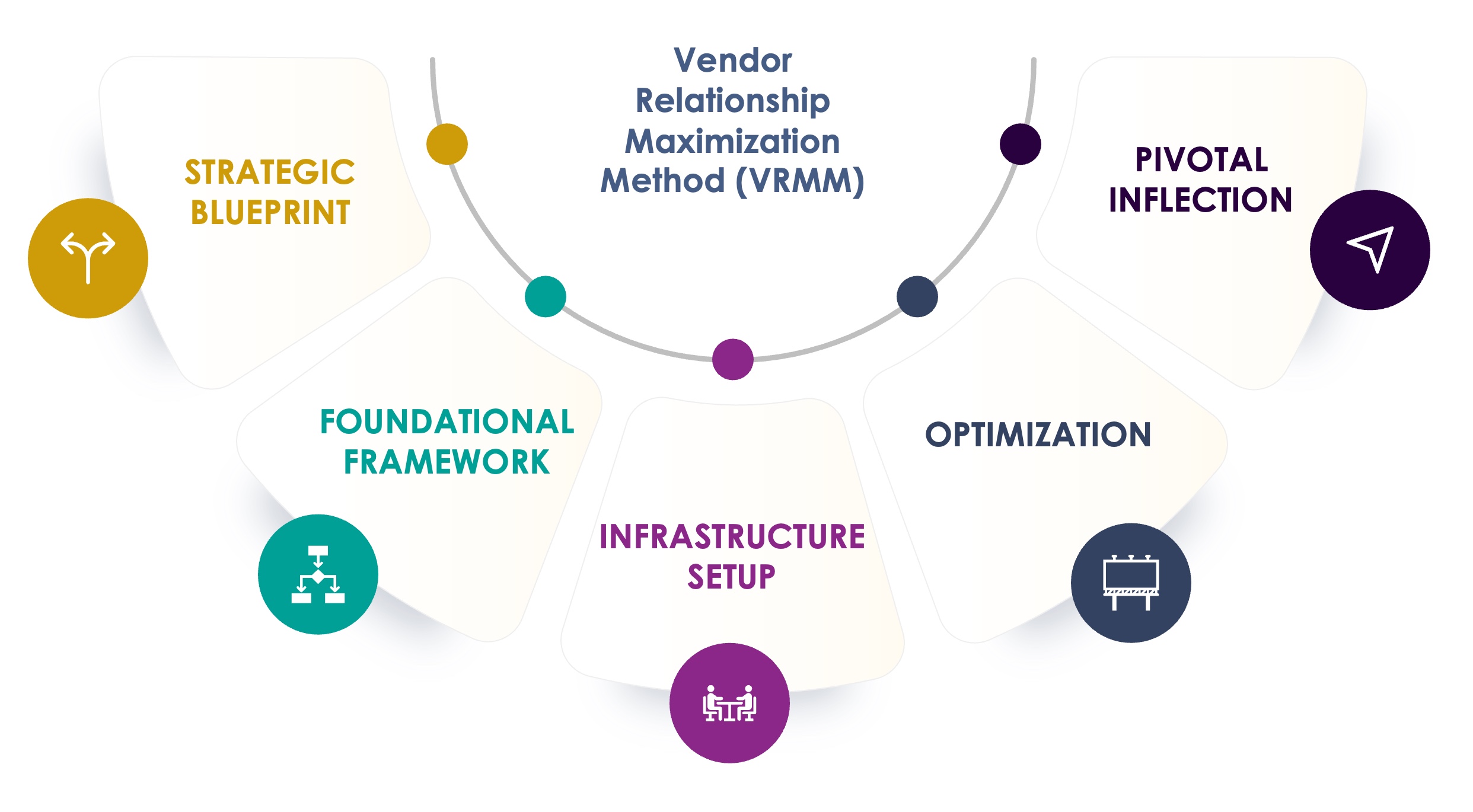
Develop a vendor strategy that aligns with your goals
- Vendor Strategy
- Market Scan
- Quality Management System (QMS)
Establish robust systems to ensure seamless vendor collaboration
- Vendor Selection
- Contract Negotiation
- Vendor Qualification (GCP & GMP)
- Systems Validation Consulting
Build an operational foundation for effective vendor management
- Study Risk Management Setup
- Governance & Reporting
- Vendor Oversight
- Clinical System Analysis
- Sponsor & Vendor Team Dynamics Workshop
- Clinical System Implementation and Oversight
Refine vendor performance to maximize efficiency and your strategic outcomes
- Vendor Management
- Budget & Change Order Management
- Risk Management
- Conflict & Collaboration Solutions
Execute key adjustments to elevate vendor partnerships and outcomes
- Inspection Readiness

