Stage 3: Infrastructure Setup
Stage 3: Infrastructure Setup is Focused on Establishing a Strong Clinical Trial Infrastructure
During the Infrastructure Setup stage, we establish robust risk management systems and clear governance structures that ensure streamlined vendor management. We safeguard your data integrity while developing governance frameworks and KPIs that drive operational excellence. Ensuring that all necessary systems and processes are in place to support stable, successful trials and maintain full regulatory compliance. With a focus on setting up a strong clinical trial infrastructure, we prepare your trials for seamless, efficient execution.

Study Risk Management Setup
Start Risk Management Early: Protect Patients, Data, and Budget
We offers a comprehensive risk management setup service to identify, assess, and mitigate potential risks in your clinical trials, ensuring compliance, patient safety, data integrity, and operational efficiency from the start. Our Study Risk Management Setup service ensures a structured approach to identifying, assessing, and mitigating risks in clinical trials. Develop a detailed risk log and facilitate ongoing risk management meetings to ensure compliance and enhance trial efficiency.
Governance & Reporting
Bespoke Governance Built with Inside Knowledge that Puts the Sponsor in Control
As a sponsor, to be compliant, you need clear oversight of your clinical trial, including the tasks and responsibilities you delegate to vendors. We have the tools you need to gain and maintain oversight throughout your clinical trial. Strengthen your oversight and solidify your governance to keep your trial on track. Take control, gain enhanced oversight, and ensure critical vendors meet the right KPIs to drive your trial forward with the comprehensive Governance.


Vendor Oversight
Comprehensive Vendor Oversight
We specialize in meticulously managing and documenting all vendors and subcontractors involved in your clinical trials. Our service provide the tools for a robust oversight approach that enhances vendor management and supports regulatory adherence.
We support you in determining and documenting the oversight you will implement for each of your vendors. We offer comprehensive vendor listings, vendor assessments, and customized vendor oversight plans. This ensures thorough documentation and effective management for all vendors, including subcontractors, to optimize oversight and regulatory compliance.
Clinical System Analysis
Protect Your Clinical Data Integrity and Safeguard your Trial
Our system and technology experts provide the independent advice you need to ensure your clinical system is fit for purpose. We map your data flow, highlighting any gaps or areas of risk to your data integrity, and explore efficiency gains through use of data integration. We quantify how integrated your clinical system is and enable you to gain a deeper understanding of the quality of data exchange and frequency. This enables you to prioritize systems that protect your data integrity and establish expectations that support you in your oversight activities.


Sponsor & Vendor Team Dynamics Workshop
Establish Cohesive Teams for Clinical Trial Success
Our Vendor and Sponsor Study Team Dynamics Workshop fosters collaboration and trust between sponsors and their critical vendor teams, ensuring cohesive team dynamics, effective communication, and the alignment necessary for successful clinical trial execution. Designed to establish a strong foundation of communication and collaboration between your clinical trial teams and your vendors. Set the stage for successful partnerships throughout the clinical trial process.
Clinical System Implementation and Oversight
Protect your Clinical Data Integrity with Expert Implementation and Oversight of your Systems
Our expert consulting team ensures seamless systems implementation and oversight from study initiation to close and archival. Through comprehensive clinical systems analysis, we provide prioritized recommendations that safeguard your interests. By facilitating communication with CROs and clinical technology vendors, we ensure efficient collaboration and alignment. With evolving regulatory requirements, We serve as your trusted partner, verifying that all systems set up by your vendors meet compliance standards and support successful trial execution.

The Seuss+ Advantage
With over 14 years of biotech and pharma experience, VRMM offers a proven, five‑step framework that enhances vendor performance, mitigates trial delays, and protects quality. We’re not passive advisors, we roll up our sleeves and execute alongside your team, ensuring hands‑on control all the way through.
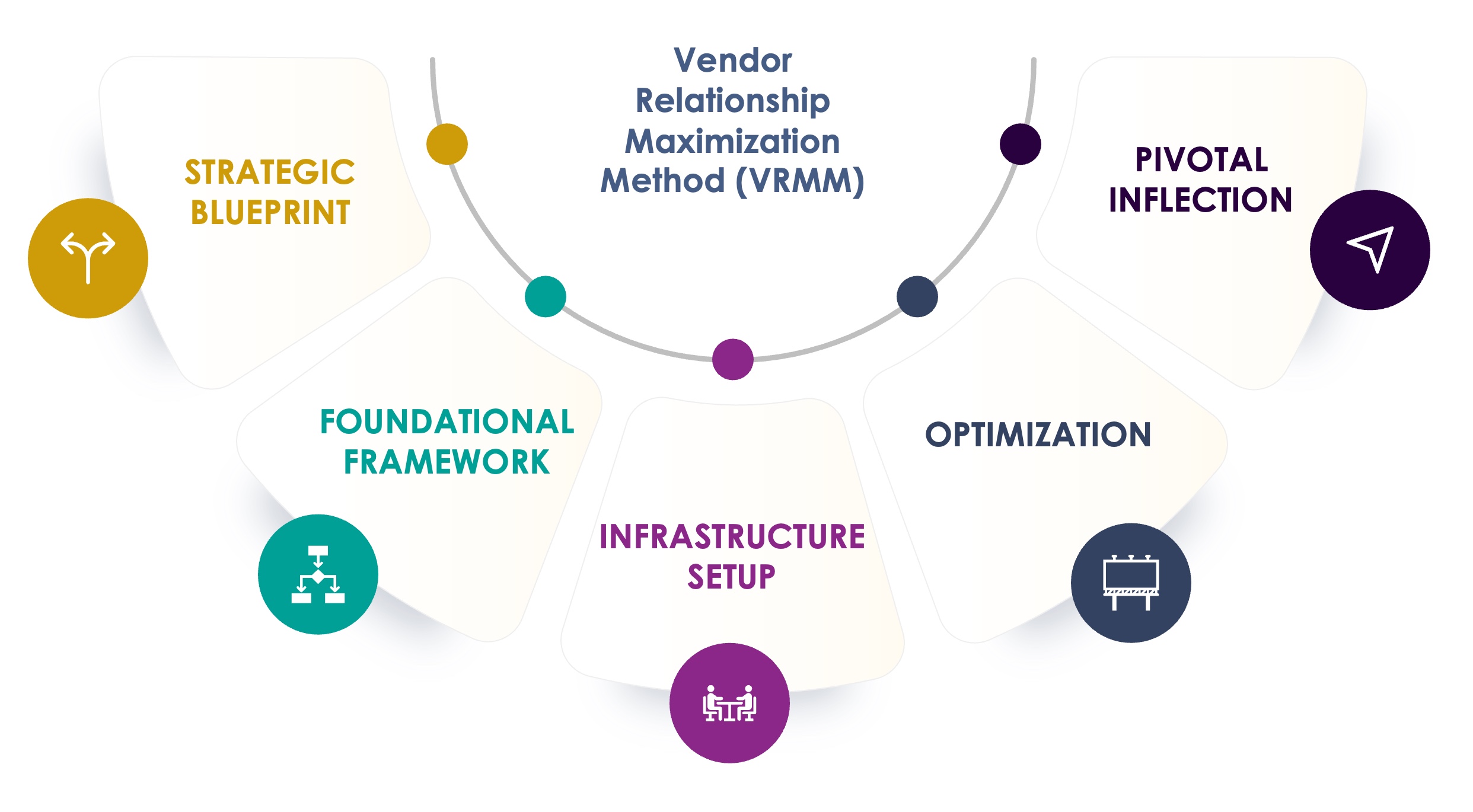
Develop a vendor strategy that aligns with your goals
- Vendor Strategy
- Market Scan
- Quality Management System (QMS)
Establish robust systems to ensure seamless vendor collaboration
- Vendor Selection
- Contract Negotiation
- Vendor Qualification (GCP & GMP)
- Systems Validation Consulting
Build an operational foundation for effective vendor management
- Study Risk Management Setup
- Governance & Reporting
- Vendor Oversight
- Clinical System Analysis
- Sponsor & Vendor Team Dynamics Workshop
- Clinical System Implementation and Oversight
Refine vendor performance to maximize efficiency and your strategic outcomes
- Vendor Management
- Budget & Change Order Management
- Risk Management
- Conflict & Collaboration Solutions
Execute key adjustments to elevate vendor partnerships and outcomes
- Inspection Readiness

