Stage 5: Pivotal Inflection
Stage 5: Pivotal Inflection Guides You Through Critical Pivotal Milestones in Clinical Trials
In the Pivotal Inflection stage, Seuss+ becomes your strategic partner, guiding you through the final critical pivotal milestones in clinical trials. We conduct thorough inspection readiness assessments, including detailed internal documentation reviews and mock regulatory inspections, ensuring all gaps are identified and addressed early. Whether your next goal is securing financial milestones, obtaining regulatory approvals, achieving clinical trial success, or navigating commercialization, mergers, or acquisitions, we tailor our support to ensure you’re fully prepared.

Inspection Readiness
Your Path to Good Quality Regulatory Submission
Our Inspection Readiness service helps you prepare for regulatory inspections by conducting thorough assessments or mock inspections led by experienced auditors simulating FDA or EMA inspectors. We manage every detail, from organizing multi-day inspection readiness meetings, setting the agenda, and coordinating attendees to handling travel logistics and venue setup. We also ensure both the front room (auditor’s workspace) and backroom (document handling and follow-ups) are fully prepared for seamless operations, enabling prompt responses and detailed tracking. By identifying potential compliance gaps early, we minimize risks and help ensure a smooth regulatory submission process.
The Seuss+ Advantage
The Seuss+ Vendor Relationship Maximization Method (VRMM) is a proven process, honed over more than a decade, that ensures your vendors perform at their best. A systematic approach that helps reduce trial delays, mitigate risks, and drive your clinical development success.
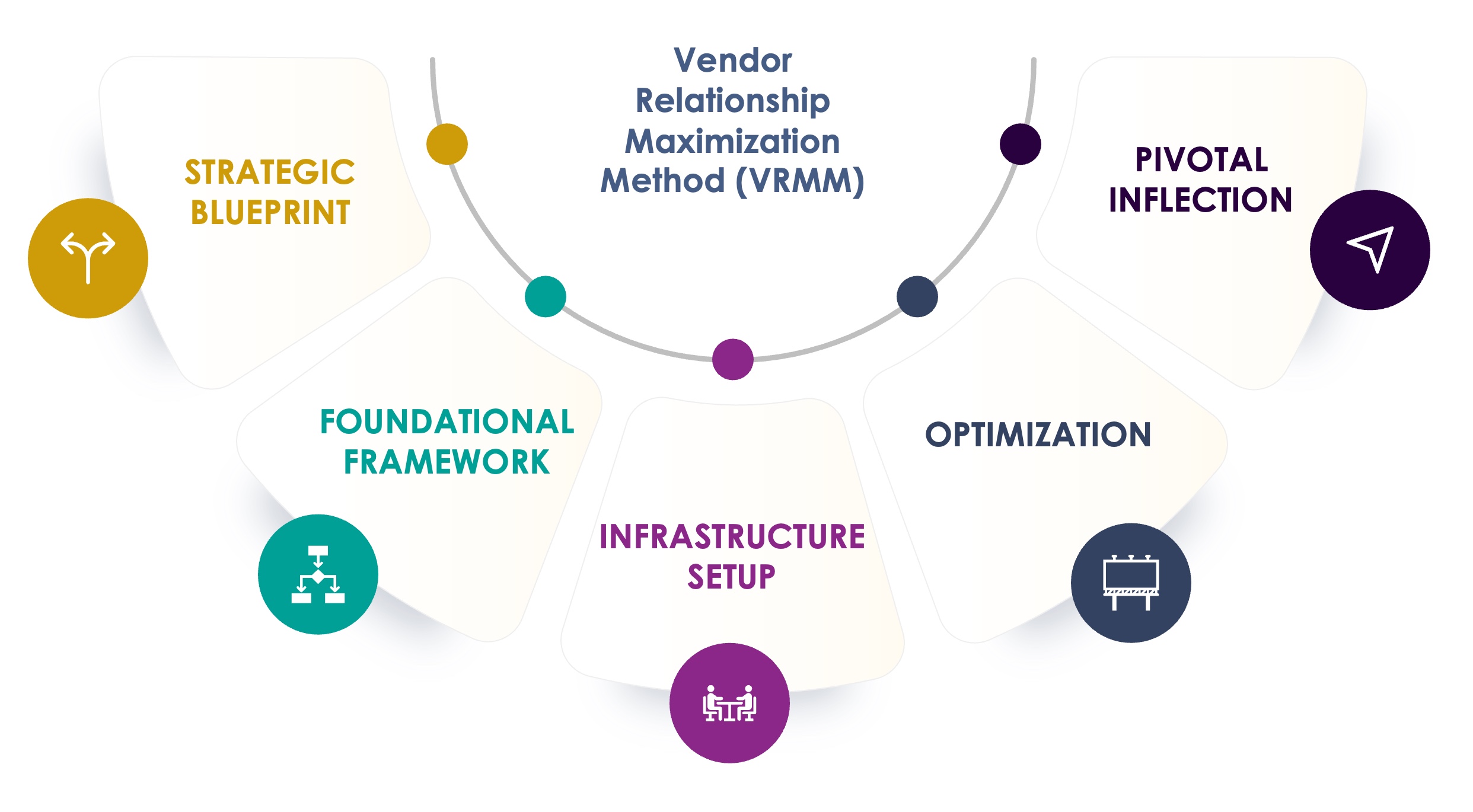
Develop a vendor strategy that aligns with your goals
- Vendor Strategy
- Market Scan
- Quality Management System (QMS)
Establish robust systems to ensure seamless vendor collaboration
- Vendor Selection
- Contract Negotiation
- Vendor Qualification (GCP & GMP)
- Systems Validation Consulting
Build an operational foundation for effective vendor management
- Study Risk Management Setup
- Governance & Reporting
- Vendor Oversight
- Clinical System Analysis
- Sponsor & Vendor Team Dynamics Workshop
- Clinical System Implementation and Oversight
Refine vendor performance to maximize efficiency and your strategic outcomes
- Vendor Management
- Budget & Change Order Management
- Risk Management
- Conflict & Collaboration Solutions
Execute key adjustments to elevate vendor partnerships and outcomes
- Inspection Readiness

